Hi all,
In this post I will tell you about a new tech that is coming up adsorption refrigeration (not absorption)
The conventional air conditioning system in automobiles uses a compressor to deliver the vapour refrigerant at high temperature to the condenser unit. The compressor derives its power from the engine itself. The cooling effect is produced by the sub cooling of the refrigerant in the condenser. Conventional vapor compression refrigeration systems, however, have a number of shortcomings which make it unviable in automobiles and there is a need for a better alternative. One of the alternatives being developed at present is adsorption refrigeration system. Adsorption is the phenomenon in which, the gas molecules (adsorbate) in the adsorbing pair gets deposited on the solid (adsorbent) surface. This is an exothermic process. Adsorption refrigeration system uses the process of adsorption-desorption and the thermodynamics associated with it to create the refrigeration cycle.
Solid vapor adsorption is similar to liquid vapor absorption system, except that the refrigerant is adsorbed at the surface of another solid known as adsorbent. A report, presented by Metrons transportation centre suggests the suitability of Adsorption systems for vehicles (Christry, 2001). In that report the performance of VCR system and VAR system has been compared. Series of carried experimental results suggest about the feasibility of adsorption systems and NH3 - activated carbon has been suggested as refrigerant adsorbent pair. “Wang et al (2009) after comparing the performances of various suitable systems has claimed that adsorption system is more suitable for automobile cooling”.
“Saha et al (2003) in the presented work have demonstrated dual mode silica gel water adsorption chillers design along with various temperature ranges and obtained optimum results for temperature range of 50 OC and 55 OC. Comparison of COP has been presented for three stage mode and single stage multiple modes. Simulation has been presented and the COP is in the range of 0.2 and 0.45 respectively”, “Wang and Oliveira (2005) have presented the achievements in solid sorption refrigeration prototypes, obtained since the interest in sorption system was renewed at the end of 1970s. The applications included ice making and air conditioning”, “Wang (2005) in his work claimed to obtain COP of 0.15 for solar application. In the review work the details of performance of adsorption system for different applications with COP has been presented in a tabular form. The COP is in the range of 0.3 to 0.6”. “Kong et al (2005) have presented an experimental investigation of the performance of a micro combined, cooling heating and power system driven by a gas engine. In the described system a COP of 0.3 for refrigeration at 13OC has been obtained successfully. The suggested system can supply electricity of 12 kW and heat load of 28 kW and cooling load of 9 kW simultaneously”, “Maggio et al (2006) presents the results of a predictive two dimensional mathematical model of an adsorption cooling machine consisting of a double consolidated adsorbent bed with internal heat recovery. Internal heat recovery enhances the COP”. It is suggested that the adsorbent thickness should be limited to 2 to 3 mm for optimum results.
“Lambert and Jones (2006) has presented a detailed study of vapor adsorption refrigeration system specifically for automobile air conditioning. After a detailed review of adsorption refrigeration, it has been suggested that activated carbon and NH3 can be the best adsorbent refrigerant pair for the selected application. Adsorption cooling has been compared with other heat generated cooling technologies and it is claimed that adsorption system is the best alternative in terms of size and mass.” In the second part of his work he has demonstrated air conditioner for a car successfully. The detailed design of the critical components has also been presented. It has been claimed that the overall weight of the system is ~3.5 percent to the total vehicle mass, which is at par with the mass of current systems. Similar work has been done to demonstrate a cooling system for a car developing 2 kW cooling power with COP 0.22, bed thickness ( δad ) of 4 mm and an eight way valve has been suggested in this work (Tamainot et al, 2008 ). “Wang et al (2006) has presented a design of an adsorption air conditioner for locomotive driver cabin, powered by 350 OC - 450 OC exhaust gases. The cooling power and COP is 5 KW and 0.25 respectively”. The cycle time of 1060 s with exhaust temperature of 450C cooling air temp of 40 OC and chilled water temp. of 10 OC is achieved. The specific cooling power of 164 W/kg to 200 W/kg has been obtained. “Huguess, and Beyene (2008) has presented an approach for the heat recovery from the automotive engine to improve COP”. Some innovations like use of fuzzy controller, heat pipe, additives in adsorber bed, suction pump with adsorber have been demonstrated in some papers for the enhancement of enhance the performance of adsorption cooling systems (Farzaneh, and Tootoonchi 2008), (Fadar et al 2009), (Tassou et al ,2009), (Hirot et al,2008).
An adsorption compressor can use thermal compression powered by the “free energy” of exhaust heat and not affect the vehicle's engine performance. Since moving parts in an adsorption system are limited to valves; it is considerably simpler, requires no lubrication, and thus little maintenance. Other advantages include quiet operation, energy efficiency and potential modularity of the system to adjust the heating and cooling capacity by the addition or subtraction of sorbent beds.
The system takes advantages of the ability of sorbent material to adsorb a relatively large quantity of refrigerant vapor (adsorbate) at low temperature and pressure and desorbs the refrigerant at a higher temperature and pressure. The compressor effect is generated by cyclically heating and cooling the sorbent material and refrigerant resulting in high pressure outward flow or refrigerant release during the hot desorption phase, and inward flow or low pressure suction during the cold adsorption phase.
Various Adsorption Processes And Pairs Available
The adsorption process is divided into these two types:
a. Physical adsorption
b. Chemical adsorption
a. Physical adsorption: It is caused by van der Walls forces between the molecules of the adsorbent and the adsorbate. Physical adsorbents with mesopores can adsorb consecutives layers of adsorbate, while those with micropores have the volume of the pores filled with the adsorbate.
Physical adsorbents develop the selectivity to the adsorbate after the former undergo specific treatments, like react under a gas stream or with certain agents. The kind of treatment will depend on the type of sorbents.
b. Chemical adsorption: It is caused by the reaction between adsorbate and the surface molecules of adsorbent. Electron transfer, atom rearrangement and fracture or formation of chemical bond always occurs in the process of chemical adsorption. Only one layer of adsorbate reacts with the surface molecules of chemical adsorbent. The adsorbate and adsorbent molecules after adsorption never keep their original state.
Moreover, there are the phenomena of salt swelling and agglomeration, which are critical for heat and mass transfer performance.
Adsorbents can be classified into the following three types:
A. Physical adsorbents
B. Chemical adsorbents
C. Composite adsorbents
A. Physical Adsorbents
The common physical adsorbents for adsorption refrigeration are activated carbon, activated carbon fibre, silica gel and zeolite.
1. Activated carbon
Activated charcoal forms an adsorption pair with methanol or ammonia. Activated carbon/methanol is one of the most common working pair due to the large adsorption quantity and lower adsorption heat, which is about 1800–2000 kJ/kg. As the main heat consumption in desorption phase is due to the adsorption heat, low values of adsorption heat are beneficial to the coefficient of performance. Also activated carbon/ammonia is a prominent adsorption pair as activated carbon can adsorb 62 % ammonia and low heat of adsorption.
Activated carbon is made of materials such as wood, peat, coal, fossil oil, chark, bone, coconut shell and nut stone. The microcrystal for the activated carbon produced from bone is a six element carboatomic ring, and the adsorption performance is influenced by the functional group that is connected to the carboatomic ring. For example, arene group increases adsorption, while sulfonic group decreases it.
2. Silica Gel
Silica Gel forms an adsorption pair with water. The adsorption heat for this pair is about 2500 kJ/kg. Desorption temperature can be very low, but above 50 °C. Desorption temperature cannot be higher than 120 °C, and it is generally lower than 90 °C. The silica gel is a type of amorphous synthetic silica. It is a rigid, continuous net of colloidal silica, connected to very small grains of hydrated SiO4. Activated silica gel exhibit a great affinity for methanol adsorbing up to 50 % by mass, much greater than its affinity for water (33 %) and ammonia (13 %).
The hydroxyl in the structure is the adsorption center because it is a polar and can form hydrogen bonds with polar oxides, such as water and alcohol. The adsorption ability of silica gel increases when the polarity increases. One hydroxyl can adsorb one molecule of water.
3. Zeolite
Zeolites are alkali aluminosilicate minerals containing myriad nanopores in their open cage like crystalline lattice which permit them to adsorb large amount of small polar molecules. Zeolite forms a pair with water. The adsorption heat for zeolite/water pair is higher than that of silica gel/water pair, and it is about 3300–4200 kJ/kg. The adsorption ability of zeolites is related to the proportion between Si and Al, and the adsorption ability is higher when this proportion is small. The adsorption and desorption heat of zeolite pairs are high, and the desorption temperature of these pairs is also high, and about 250– 300°C. Most zeolite molecular sieves can be destructed at temperatures higher than 600–700 °C. Zeolites have low k of the order of 0.1-1.0 W / m K which slows adsorption and desorption, thereby limiting the specific cooling power (SCP). Zeolite can adsorb 36, 22, and 30 weight % water, ammonia and methanol respectively.
B. Chemical adsorbents
Chemical adsorbents mainly include metal chlorides, metal hydrides and metal oxides.
C. Composite Adsorbents
Composite adsorbents started to be studied about 20 years ago, and they aimed to improve the heat and mass transfer performance of the original chemical adsorbents. This kind of adsorbent is usually obtained by the combination of a chemical adsorbent and a porous medium, that can be or not a physical adsorbent, such as activated carbon, graphite, carbon fibre, etc.
Following refrigerants can be used in the adsorption refrigeration system:
1. Water:
It is non-toxic, non-flammable, non-polluting, stable and has the highest latent heat among common substances (hfg = 2257 kJ/kg at Patm). However, its vapor pressure is very low (Pcond = 25 kPa at 65 ᴼC, Pevap = 0.8 kPa at 3 ᴼC), requiring a large condenser and evaporator. Moreover, operating at sub atmospheric pressure invites air „poisoning‟. Operating the evaporator at just a few degrees above freezing point requires precise control and the tubing must be drained to prevent bursting when idle in cold weather.
2. Ammonia:
Ammonia is flammable in some concentrations (16-25 %), non-polluting, stable, and has the second highest latent heat (hfg = 1368 kJ/kg at Patm) among common substances. When throttled (isentropic) from a liquid at at Tcond.out= 60⁰C to Tevap.in= -10⁰C, Δhevap= 958 kJ/kg. Ammonia has Pcond.in= 2948 kPa at 65 ⁰C and Pevap.out= 291 kPa at -13⁰C.
3. Methanol:
It is toxic, highly inflammable, non-polluting, unstable beyond 393 K, and has the third highest latent heat (hfg = 1101 J/kg at Patm) among common substances. When throttled from 60 ᴼC to -10 ᴼC, Δhevap = 1000 kJ/kg. Methanol has Pcond.in= 101 kPa at 65 ᴼC but Pevap.out ≈ 10 kPa at -13⁰C, so, „poisoning‟ by air is possibility.
4. Ethanol:
Ethanol is similar to methanol, but is surpassed by methanol in all relevant thermo physical properties. Ethanol also has sub atmospheric operating pressures.
5. Propane:
Propane is relatively non-toxic (irritating at high concentration, but can asphyxiate if it displaces too much O2), highly inflammable, and non-polluting. It has practical operating pressures (>Patm) but thermal properties are inferior to those of H2O, NH3, and CH3OH.
6. Carbon dioxide:
CO2 is also being explored by some European automakers. It is non-toxic, non-flammable, and non-polluting but requires very high Pcond ≈ 7000 kPa, requiring a thick walled adsorber shell and tubing.
Adsorption refers to the binding of molecules (sorbate) to surface of an inert material (sorbent) without any chemical change. Adsorption is better described as a physical process at surface as opposed to take up or in by molecular or chemical action such as absorption. Adsorption occurs because the atoms, molecular or ions at the surface of the sorbent are extremely reactive with unfulfilled valence requirements as compared to their counterparts in the interior, which have valence requirements satisfied. The unused bonding capacity of surface atoms may be utilized to bond molecules of the sorbate to the surface of sorbent. It uses clathrate material. A clathrate is an organic compound that has 3-dimentional lattices that make up a network of micro pores or individual sites for the sorbate to reside. The sorbate, while attached to the sorbent surface, gets trapped in cavities of the sorbents‟ cage like crystals.
The adsorption capacity is a function of the certain characteristics of sorbate and sorbent such as; sorbent porosity, sorbate boiling point, and operating temperature and pressure. Maximizing the adsorption capacity is optimal as it allows for smaller packing sizes for given cooling capacity. Activated carbon is optimal as it allows for smaller packing sizes for given cooling capacity. Activated carbon is an excellent sorbent material because tarry carbonization products have been removed for the pores – resulting in a large surface area for interaction throughout the material. Conventional refrigerants such as R-134a and R-717 (ammonia) are readily available and perform in the desired temperature and pressure regions when used in an activated carbon adsorption system.
Solid – vapor adsorption is similar to liquid-vapor absorption, except that the refrigerant is adsorbed onto a solid surface rather than absorbed into a liquid. The adsorption cycle is illustrated in Fig. 1.4.1 and processes are as follows.
Process 1-2
1. At state 1, a cool adsorber contains adsorbent saturated with a large fraction of refrigerant at slightly below Pevap. The cool adsorber is heated and desorbs refrigerant vapor isosterically (i.e. at constant total mass in the adsorber), thereby it to state 2 slightly above Pcond. At this point, vapor starts being forced out of the hot adsorber through a one-way valve to the condenser.
Process 2-3
2. Isobaric heating desorbs more refrigerant, forcing it into the condenser until state 3 attained, where the adsorber is nearly devoid of refrigerant.
Process 3-4
3. The hot adsorber is then cooled isosterically (at constant total mass), causing adsorption and depressurization, until the pressure drops below Pevap (state4), opening another check valve to allow vapor to enter the adsorber from the evaporator.
Process 4-1
4. Isobaric cooling to state 1 saturates the adsorbent, completing the cycle.
REFERENCES
1. A. C. Deshpande, R. M. Pillai, (2009), „Adsorption Air-Conditioning (AdAC) for Automobiles Using Waste Heat Recovered from Exhaust Gases‟, Second International Conference on Emerging Trends in Engineering and Technology, ICETET-09
2. Craig Christy and Reza Toossi, August 5, 2004, „Adsorption Air Conditioning for Containerships and Vehicles‟, Metrans Report 00-7; California State University Long Beach.
3. Li Yong* and Ruzhu Z. Wang, (2007), „Adsorption Refrigeration: A Survey of Novel Technologies‟, Recent Patents on Engineering 2007, Vol. 1, No. 1, pp.1-21.
4. M. A. Lambert and B. J. Jones, (2006) „Automotive adsorption air conditioner powered by exhaust heat. Part 1: conceptual and embodiment design‟ J. Automobile Engineering, Part D, Vol. 220, pp.959-972.
5. M. A. Lambert and B. J. Jones, (2006) „Automotive adsorption air conditioner powered by exhaust heat. Part 2: Detailed design and analysis‟ J. Automobile Engineering, Part D, Vol. 220, pp.973-989.
6. M. L. Mathur, F. S. Mehta, (2010), „Refrigerant and Psychometric Properties‟, Jain Brothers.
7. R. K. Rajput, (2009), „Heat and Mass Transfer‟, S. Chand and Company Ltd.
8. V.B. Bhandari (2009) „Design of machine elements‟ Tata Mcgraw Hill,Third edition,2009.
9. V. M. Domkundwar, (2010), „Heat and Mass Transfer Data Book‟, DP Rai & Co.
10. Zhong Yongfang, Wert Kevin L., and Fang Tiegang, (2010) „An Adsorption Air-Conditioning System to Reduce Engine Emissions and Fuel Consumption for Heavy-Duty Vehicles‟. International Refrigeration and Air Conditioning Conference, Paper 1100.
In this post I will tell you about a new tech that is coming up adsorption refrigeration (not absorption)
INTRODUCTION
The conventional air conditioning system in automobiles uses a compressor to deliver the vapour refrigerant at high temperature to the condenser unit. The compressor derives its power from the engine itself. The cooling effect is produced by the sub cooling of the refrigerant in the condenser. Conventional vapor compression refrigeration systems, however, have a number of shortcomings which make it unviable in automobiles and there is a need for a better alternative. One of the alternatives being developed at present is adsorption refrigeration system. Adsorption is the phenomenon in which, the gas molecules (adsorbate) in the adsorbing pair gets deposited on the solid (adsorbent) surface. This is an exothermic process. Adsorption refrigeration system uses the process of adsorption-desorption and the thermodynamics associated with it to create the refrigeration cycle.
LITERATURE AVAILABLE
Solid vapor adsorption is similar to liquid vapor absorption system, except that the refrigerant is adsorbed at the surface of another solid known as adsorbent. A report, presented by Metrons transportation centre suggests the suitability of Adsorption systems for vehicles (Christry, 2001). In that report the performance of VCR system and VAR system has been compared. Series of carried experimental results suggest about the feasibility of adsorption systems and NH3 - activated carbon has been suggested as refrigerant adsorbent pair. “Wang et al (2009) after comparing the performances of various suitable systems has claimed that adsorption system is more suitable for automobile cooling”.
“Saha et al (2003) in the presented work have demonstrated dual mode silica gel water adsorption chillers design along with various temperature ranges and obtained optimum results for temperature range of 50 OC and 55 OC. Comparison of COP has been presented for three stage mode and single stage multiple modes. Simulation has been presented and the COP is in the range of 0.2 and 0.45 respectively”, “Wang and Oliveira (2005) have presented the achievements in solid sorption refrigeration prototypes, obtained since the interest in sorption system was renewed at the end of 1970s. The applications included ice making and air conditioning”, “Wang (2005) in his work claimed to obtain COP of 0.15 for solar application. In the review work the details of performance of adsorption system for different applications with COP has been presented in a tabular form. The COP is in the range of 0.3 to 0.6”. “Kong et al (2005) have presented an experimental investigation of the performance of a micro combined, cooling heating and power system driven by a gas engine. In the described system a COP of 0.3 for refrigeration at 13OC has been obtained successfully. The suggested system can supply electricity of 12 kW and heat load of 28 kW and cooling load of 9 kW simultaneously”, “Maggio et al (2006) presents the results of a predictive two dimensional mathematical model of an adsorption cooling machine consisting of a double consolidated adsorbent bed with internal heat recovery. Internal heat recovery enhances the COP”. It is suggested that the adsorbent thickness should be limited to 2 to 3 mm for optimum results.
“Lambert and Jones (2006) has presented a detailed study of vapor adsorption refrigeration system specifically for automobile air conditioning. After a detailed review of adsorption refrigeration, it has been suggested that activated carbon and NH3 can be the best adsorbent refrigerant pair for the selected application. Adsorption cooling has been compared with other heat generated cooling technologies and it is claimed that adsorption system is the best alternative in terms of size and mass.” In the second part of his work he has demonstrated air conditioner for a car successfully. The detailed design of the critical components has also been presented. It has been claimed that the overall weight of the system is ~3.5 percent to the total vehicle mass, which is at par with the mass of current systems. Similar work has been done to demonstrate a cooling system for a car developing 2 kW cooling power with COP 0.22, bed thickness ( δad ) of 4 mm and an eight way valve has been suggested in this work (Tamainot et al, 2008 ). “Wang et al (2006) has presented a design of an adsorption air conditioner for locomotive driver cabin, powered by 350 OC - 450 OC exhaust gases. The cooling power and COP is 5 KW and 0.25 respectively”. The cycle time of 1060 s with exhaust temperature of 450C cooling air temp of 40 OC and chilled water temp. of 10 OC is achieved. The specific cooling power of 164 W/kg to 200 W/kg has been obtained. “Huguess, and Beyene (2008) has presented an approach for the heat recovery from the automotive engine to improve COP”. Some innovations like use of fuzzy controller, heat pipe, additives in adsorber bed, suction pump with adsorber have been demonstrated in some papers for the enhancement of enhance the performance of adsorption cooling systems (Farzaneh, and Tootoonchi 2008), (Fadar et al 2009), (Tassou et al ,2009), (Hirot et al,2008).
ADSORPTION REFRIGERATION SYSTEM
An adsorption compressor can use thermal compression powered by the “free energy” of exhaust heat and not affect the vehicle's engine performance. Since moving parts in an adsorption system are limited to valves; it is considerably simpler, requires no lubrication, and thus little maintenance. Other advantages include quiet operation, energy efficiency and potential modularity of the system to adjust the heating and cooling capacity by the addition or subtraction of sorbent beds.
The system takes advantages of the ability of sorbent material to adsorb a relatively large quantity of refrigerant vapor (adsorbate) at low temperature and pressure and desorbs the refrigerant at a higher temperature and pressure. The compressor effect is generated by cyclically heating and cooling the sorbent material and refrigerant resulting in high pressure outward flow or refrigerant release during the hot desorption phase, and inward flow or low pressure suction during the cold adsorption phase.
Various Adsorption Processes And Pairs Available
The adsorption process is divided into these two types:
a. Physical adsorption
b. Chemical adsorption
a. Physical adsorption: It is caused by van der Walls forces between the molecules of the adsorbent and the adsorbate. Physical adsorbents with mesopores can adsorb consecutives layers of adsorbate, while those with micropores have the volume of the pores filled with the adsorbate.
Physical adsorbents develop the selectivity to the adsorbate after the former undergo specific treatments, like react under a gas stream or with certain agents. The kind of treatment will depend on the type of sorbents.
b. Chemical adsorption: It is caused by the reaction between adsorbate and the surface molecules of adsorbent. Electron transfer, atom rearrangement and fracture or formation of chemical bond always occurs in the process of chemical adsorption. Only one layer of adsorbate reacts with the surface molecules of chemical adsorbent. The adsorbate and adsorbent molecules after adsorption never keep their original state.
Moreover, there are the phenomena of salt swelling and agglomeration, which are critical for heat and mass transfer performance.
Adsorbents can be classified into the following three types:
A. Physical adsorbents
B. Chemical adsorbents
C. Composite adsorbents
A. Physical Adsorbents
The common physical adsorbents for adsorption refrigeration are activated carbon, activated carbon fibre, silica gel and zeolite.
1. Activated carbon
Activated charcoal forms an adsorption pair with methanol or ammonia. Activated carbon/methanol is one of the most common working pair due to the large adsorption quantity and lower adsorption heat, which is about 1800–2000 kJ/kg. As the main heat consumption in desorption phase is due to the adsorption heat, low values of adsorption heat are beneficial to the coefficient of performance. Also activated carbon/ammonia is a prominent adsorption pair as activated carbon can adsorb 62 % ammonia and low heat of adsorption.
Activated carbon is made of materials such as wood, peat, coal, fossil oil, chark, bone, coconut shell and nut stone. The microcrystal for the activated carbon produced from bone is a six element carboatomic ring, and the adsorption performance is influenced by the functional group that is connected to the carboatomic ring. For example, arene group increases adsorption, while sulfonic group decreases it.
2. Silica Gel
Silica Gel forms an adsorption pair with water. The adsorption heat for this pair is about 2500 kJ/kg. Desorption temperature can be very low, but above 50 °C. Desorption temperature cannot be higher than 120 °C, and it is generally lower than 90 °C. The silica gel is a type of amorphous synthetic silica. It is a rigid, continuous net of colloidal silica, connected to very small grains of hydrated SiO4. Activated silica gel exhibit a great affinity for methanol adsorbing up to 50 % by mass, much greater than its affinity for water (33 %) and ammonia (13 %).
The hydroxyl in the structure is the adsorption center because it is a polar and can form hydrogen bonds with polar oxides, such as water and alcohol. The adsorption ability of silica gel increases when the polarity increases. One hydroxyl can adsorb one molecule of water.
3. Zeolite
Zeolites are alkali aluminosilicate minerals containing myriad nanopores in their open cage like crystalline lattice which permit them to adsorb large amount of small polar molecules. Zeolite forms a pair with water. The adsorption heat for zeolite/water pair is higher than that of silica gel/water pair, and it is about 3300–4200 kJ/kg. The adsorption ability of zeolites is related to the proportion between Si and Al, and the adsorption ability is higher when this proportion is small. The adsorption and desorption heat of zeolite pairs are high, and the desorption temperature of these pairs is also high, and about 250– 300°C. Most zeolite molecular sieves can be destructed at temperatures higher than 600–700 °C. Zeolites have low k of the order of 0.1-1.0 W / m K which slows adsorption and desorption, thereby limiting the specific cooling power (SCP). Zeolite can adsorb 36, 22, and 30 weight % water, ammonia and methanol respectively.
B. Chemical adsorbents
Chemical adsorbents mainly include metal chlorides, metal hydrides and metal oxides.
C. Composite Adsorbents
Composite adsorbents started to be studied about 20 years ago, and they aimed to improve the heat and mass transfer performance of the original chemical adsorbents. This kind of adsorbent is usually obtained by the combination of a chemical adsorbent and a porous medium, that can be or not a physical adsorbent, such as activated carbon, graphite, carbon fibre, etc.
Following refrigerants can be used in the adsorption refrigeration system:
1. Water:
It is non-toxic, non-flammable, non-polluting, stable and has the highest latent heat among common substances (hfg = 2257 kJ/kg at Patm). However, its vapor pressure is very low (Pcond = 25 kPa at 65 ᴼC, Pevap = 0.8 kPa at 3 ᴼC), requiring a large condenser and evaporator. Moreover, operating at sub atmospheric pressure invites air „poisoning‟. Operating the evaporator at just a few degrees above freezing point requires precise control and the tubing must be drained to prevent bursting when idle in cold weather.
2. Ammonia:
Ammonia is flammable in some concentrations (16-25 %), non-polluting, stable, and has the second highest latent heat (hfg = 1368 kJ/kg at Patm) among common substances. When throttled (isentropic) from a liquid at at Tcond.out= 60⁰C to Tevap.in= -10⁰C, Δhevap= 958 kJ/kg. Ammonia has Pcond.in= 2948 kPa at 65 ⁰C and Pevap.out= 291 kPa at -13⁰C.
3. Methanol:
It is toxic, highly inflammable, non-polluting, unstable beyond 393 K, and has the third highest latent heat (hfg = 1101 J/kg at Patm) among common substances. When throttled from 60 ᴼC to -10 ᴼC, Δhevap = 1000 kJ/kg. Methanol has Pcond.in= 101 kPa at 65 ᴼC but Pevap.out ≈ 10 kPa at -13⁰C, so, „poisoning‟ by air is possibility.
4. Ethanol:
Ethanol is similar to methanol, but is surpassed by methanol in all relevant thermo physical properties. Ethanol also has sub atmospheric operating pressures.
5. Propane:
Propane is relatively non-toxic (irritating at high concentration, but can asphyxiate if it displaces too much O2), highly inflammable, and non-polluting. It has practical operating pressures (>Patm) but thermal properties are inferior to those of H2O, NH3, and CH3OH.
6. Carbon dioxide:
CO2 is also being explored by some European automakers. It is non-toxic, non-flammable, and non-polluting but requires very high Pcond ≈ 7000 kPa, requiring a thick walled adsorber shell and tubing.
ADSORPTION SYSTEMS WORKING PRINCIPLE
Adsorption refers to the binding of molecules (sorbate) to surface of an inert material (sorbent) without any chemical change. Adsorption is better described as a physical process at surface as opposed to take up or in by molecular or chemical action such as absorption. Adsorption occurs because the atoms, molecular or ions at the surface of the sorbent are extremely reactive with unfulfilled valence requirements as compared to their counterparts in the interior, which have valence requirements satisfied. The unused bonding capacity of surface atoms may be utilized to bond molecules of the sorbate to the surface of sorbent. It uses clathrate material. A clathrate is an organic compound that has 3-dimentional lattices that make up a network of micro pores or individual sites for the sorbate to reside. The sorbate, while attached to the sorbent surface, gets trapped in cavities of the sorbents‟ cage like crystals.
The adsorption capacity is a function of the certain characteristics of sorbate and sorbent such as; sorbent porosity, sorbate boiling point, and operating temperature and pressure. Maximizing the adsorption capacity is optimal as it allows for smaller packing sizes for given cooling capacity. Activated carbon is optimal as it allows for smaller packing sizes for given cooling capacity. Activated carbon is an excellent sorbent material because tarry carbonization products have been removed for the pores – resulting in a large surface area for interaction throughout the material. Conventional refrigerants such as R-134a and R-717 (ammonia) are readily available and perform in the desired temperature and pressure regions when used in an activated carbon adsorption system.
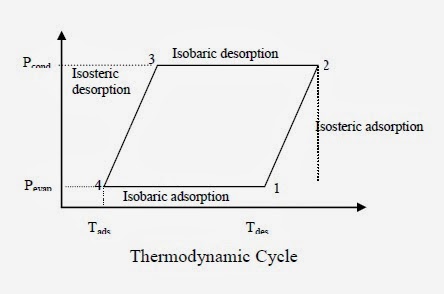
Solid – vapor adsorption is similar to liquid-vapor absorption, except that the refrigerant is adsorbed onto a solid surface rather than absorbed into a liquid. The adsorption cycle is illustrated in Fig. 1.4.1 and processes are as follows.
Process 1-2
1. At state 1, a cool adsorber contains adsorbent saturated with a large fraction of refrigerant at slightly below Pevap. The cool adsorber is heated and desorbs refrigerant vapor isosterically (i.e. at constant total mass in the adsorber), thereby it to state 2 slightly above Pcond. At this point, vapor starts being forced out of the hot adsorber through a one-way valve to the condenser.
Process 2-3
2. Isobaric heating desorbs more refrigerant, forcing it into the condenser until state 3 attained, where the adsorber is nearly devoid of refrigerant.
Process 3-4
3. The hot adsorber is then cooled isosterically (at constant total mass), causing adsorption and depressurization, until the pressure drops below Pevap (state4), opening another check valve to allow vapor to enter the adsorber from the evaporator.
Process 4-1
4. Isobaric cooling to state 1 saturates the adsorbent, completing the cycle.
REFERENCES
1. A. C. Deshpande, R. M. Pillai, (2009), „Adsorption Air-Conditioning (AdAC) for Automobiles Using Waste Heat Recovered from Exhaust Gases‟, Second International Conference on Emerging Trends in Engineering and Technology, ICETET-09
2. Craig Christy and Reza Toossi, August 5, 2004, „Adsorption Air Conditioning for Containerships and Vehicles‟, Metrans Report 00-7; California State University Long Beach.
3. Li Yong* and Ruzhu Z. Wang, (2007), „Adsorption Refrigeration: A Survey of Novel Technologies‟, Recent Patents on Engineering 2007, Vol. 1, No. 1, pp.1-21.
4. M. A. Lambert and B. J. Jones, (2006) „Automotive adsorption air conditioner powered by exhaust heat. Part 1: conceptual and embodiment design‟ J. Automobile Engineering, Part D, Vol. 220, pp.959-972.
5. M. A. Lambert and B. J. Jones, (2006) „Automotive adsorption air conditioner powered by exhaust heat. Part 2: Detailed design and analysis‟ J. Automobile Engineering, Part D, Vol. 220, pp.973-989.
6. M. L. Mathur, F. S. Mehta, (2010), „Refrigerant and Psychometric Properties‟, Jain Brothers.
7. R. K. Rajput, (2009), „Heat and Mass Transfer‟, S. Chand and Company Ltd.
8. V.B. Bhandari (2009) „Design of machine elements‟ Tata Mcgraw Hill,Third edition,2009.
9. V. M. Domkundwar, (2010), „Heat and Mass Transfer Data Book‟, DP Rai & Co.
10. Zhong Yongfang, Wert Kevin L., and Fang Tiegang, (2010) „An Adsorption Air-Conditioning System to Reduce Engine Emissions and Fuel Consumption for Heavy-Duty Vehicles‟. International Refrigeration and Air Conditioning Conference, Paper 1100.
Comments
Post a Comment